Table of contents
On buildings, tools or vehicles: metals are used as robust and durable materials in almost every area. Most metals react with oxygen forming an oxidation layer on the surface. Depending on the type of metal, this can cause the components to become unstable and brittle. You can counteract this with regular cleaning and care. In this guide, you can learn how corrosion occurs and which devices, home remedies and methods are best suited for cleaning different metals.
How do tarnished and rusting surfaces occur?
The fact that metal surfaces tarnish, discolour or rust is due to a chemical reaction called oxidation. When an element comes into contact with another element (the so-called oxidant), it gives off electrons and thus changes its state. For many metals, the oxidant is oxygen, but other chemical elements can also cause this, such as carbon, sulphur or hydrogen. Oxidation is not always harmful and cleaning oxidised metal can be relatively straightforward.
Oxidation does not always have to be harmful
Depending on the type of metal, oxidation does not necessarily have a negative effect. In the case of non-ferrous metals such as aluminium, zinc or chrome, the oxide layer even has a particularly corrosion-resistant effect, which can protect the metal and prevent further decomposition. This effect is even produced intentionally when anodising aluminium to make the components particularly durable. The same applies to copper and its alloys (e.g., bronze or brass). On these, a green patina forms upon contact with the carbon and sulphur dioxide contained in the air, which is often colloquially called verdigris. From a chemical point of view, verdigris is a name for copper acetate, which is only produced by an artificial reaction of copper and acetic acid in the laboratory. In contrast to verdigris, the naturally formed patina on copper parts is not water-soluble and effectively protects them from corrosion and wear.
Rust and its damaging effect on ferrous metals
Rust only becomes problematic when corrosion occurs. Ferrous metals react sensitively when they come into contact with water and oxygen: a porous rust develops, which decomposes the material due to its electrochemical properties. This leads to the phenomenon that rust literally “eats its way” into the iron components and permanently destroys the material in the process. So as soon as you see the first traces of rust, you should remove the rust from the metal to stop the corrosion process.
It is not only oxidation that causes metals to discolour. Water containing lime, oil and grease or soot can also trigger chemical reactions and leave unsightly stains that lead to corrosion in some metals. You can, however, easily remove these discolourations with the right metal cleaner.
What home remedies can you use to clean tarnished metal?
There are several ways you can make tarnished and dull metal shine again and remove discolouration or rust. Most methods are based on reversing the (electro-)chemical reaction that has previously taken place. Mechanical methods in which the surfaces are removed and resealed — as you probably know from wood care — cannot be used on metal, or only with a great deal of effort. But this is only necessary in the rarest of cases anyway, because treatment with a suitable metal cleaner or simple household remedies often already achieves the goal. The following table shows an overview:
| Type of metal | Cleaning agents | Procedure and instructions |
|---|---|---|
| • aluminium • copper • stainless steel | • acid-based liquid • The following household remedies are suitable: • vinegar • lemon juice • baking soda • cola | • Mix the agent of your choice with hot water. • Clean the affected surfaces generously with it. |
| • tin | • lye, e.g. water with mild soap • washing-up liquid | • Allow the liquid to act on the affected area. |
| • zinc | • water • for heavy soiling: clean-ing alcohol | • Zinc is mainly used for commodities such as garden tools or gutters. • That is why it is im-portant that the natural corrosion protection of the patina is preserved. |
| • silver | • lemon juice and salt into boiling water • Add a less noble metal to the liquid (e.g., alumini-um foil). | • Add the silver to the liquid. • Make sure there is sufficient ventila-tion, as the dis-solved sulphur oxide creates a strong odour. |
When cleaning or polishing metal, be sure to avoid cleaners with abrasive particles, hard brushes and scratchy household sponges that could leave clearly visible scratches on the delicate surfaces. It is best to use soft cotton or microfibre cleaning cloths.
Instructions: cleaning oxidised metal
With this step-by-step guide, you will learn how to clean rust off metal and prevent metal corrosion.
- Remove coarse dirt
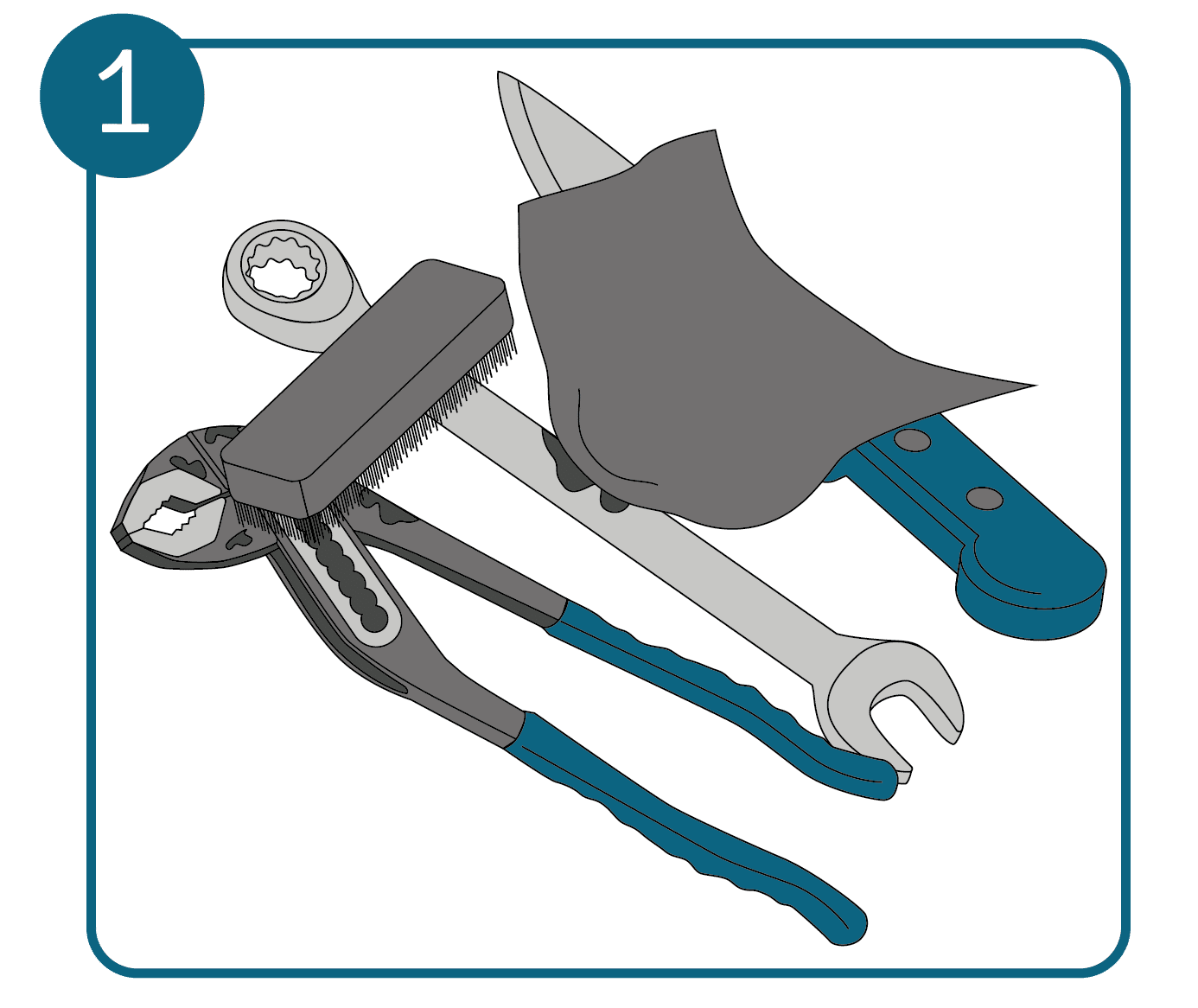 First you need to clean the metal surface. Use a soft brush or cloth to remove flash rust, dust and other loose dirt. Apply as little pressure as possible so that the particles do not leave scratches in the metal.
First you need to clean the metal surface. Use a soft brush or cloth to remove flash rust, dust and other loose dirt. Apply as little pressure as possible so that the particles do not leave scratches in the metal. - Soak oxidised metal
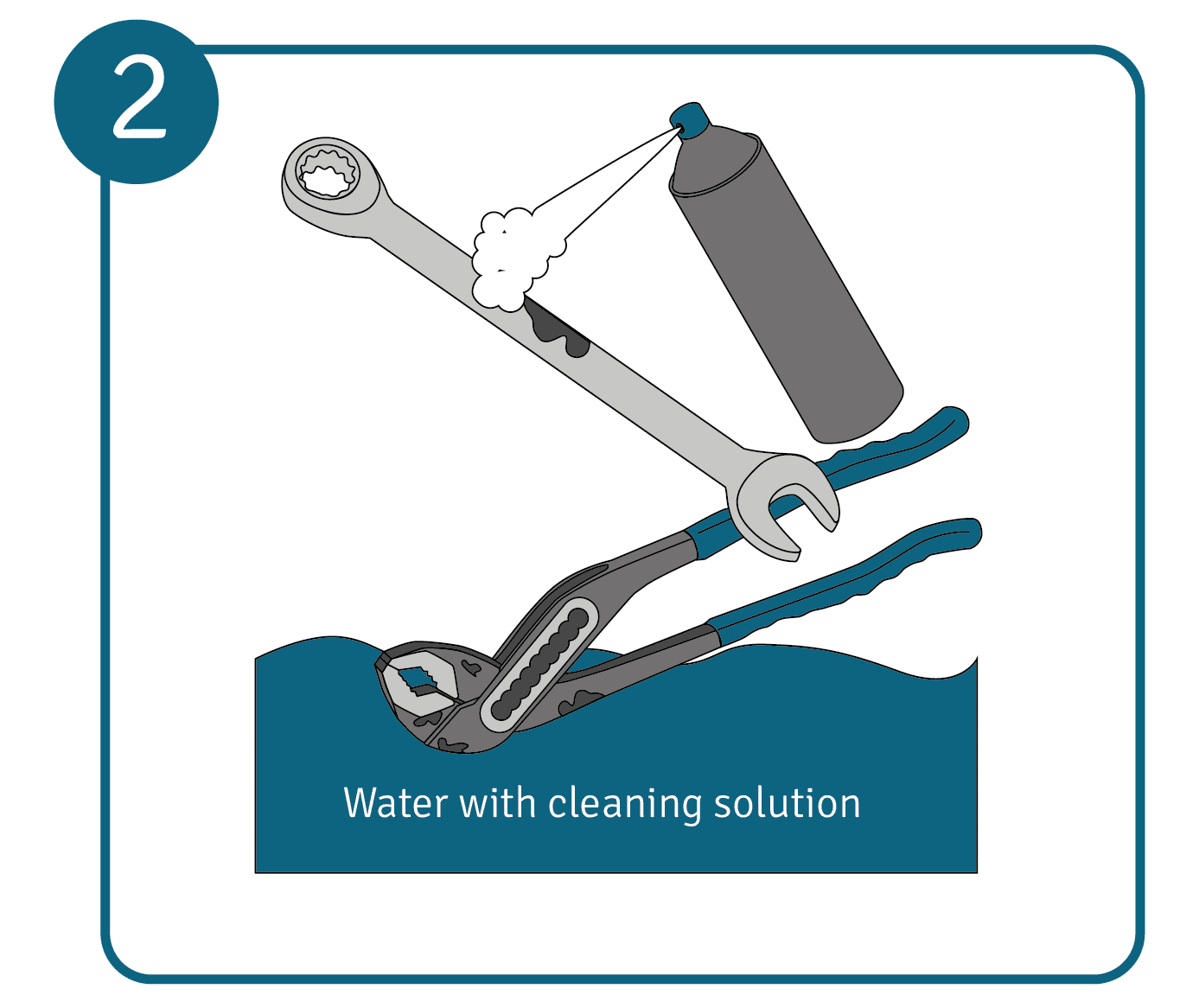 Depending on the size, generously coat or spray the oxidised areas with the cleaner of your choice or submerge the object completely in a water bath with cleaning solution. Allow the metal object to soak in the cleaning agent for about 10 to 15 minutes to completely dissolve the oxide layer.
Depending on the size, generously coat or spray the oxidised areas with the cleaner of your choice or submerge the object completely in a water bath with cleaning solution. Allow the metal object to soak in the cleaning agent for about 10 to 15 minutes to completely dissolve the oxide layer. - Rinse with clean water
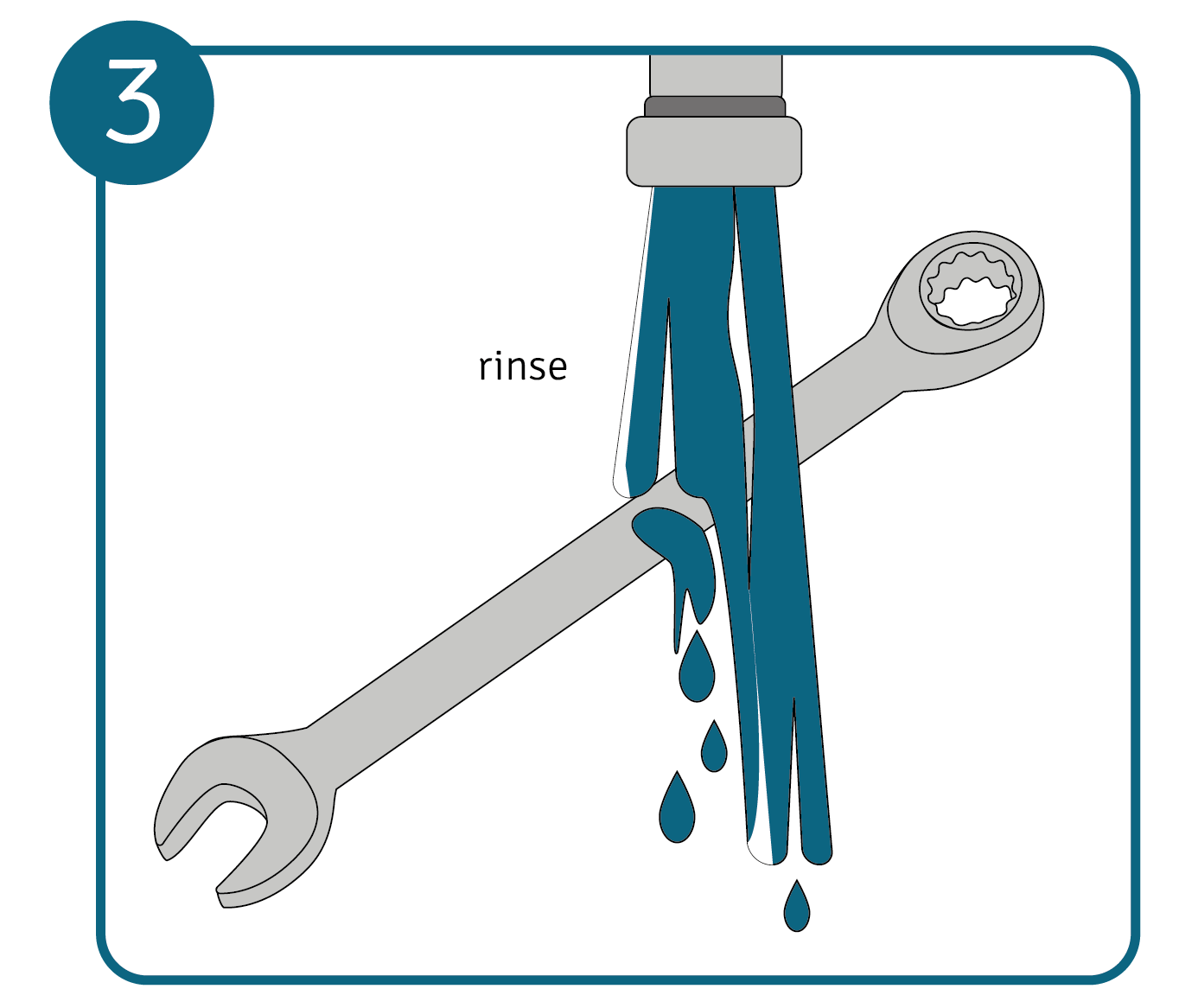 Then rinse the metal with clean water for a few minutes so that no residue of the metal cleaner remains.
Then rinse the metal with clean water for a few minutes so that no residue of the metal cleaner remains. - Clean the metal
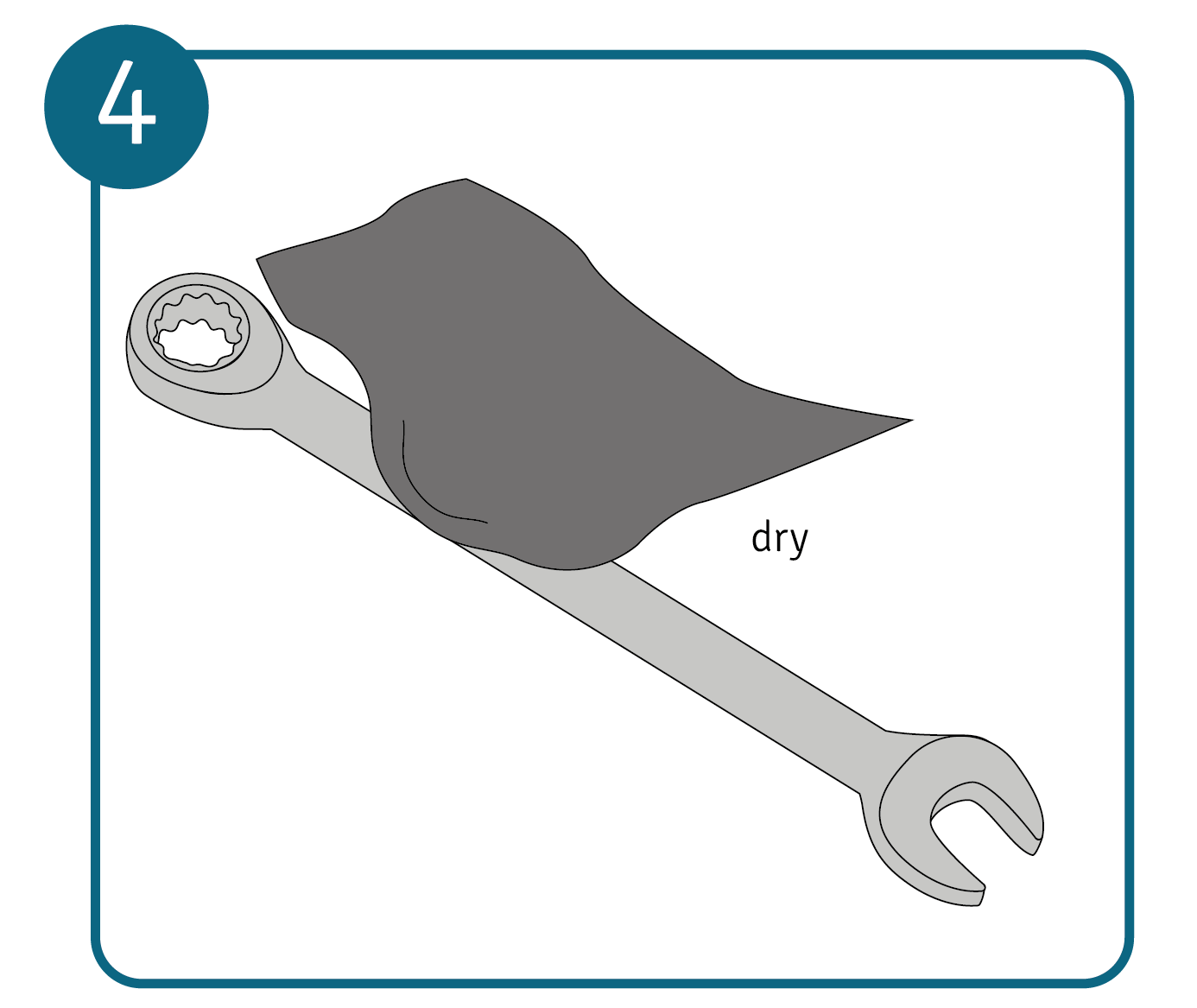 Then rub the metal dry with a suitable cloth and check whether the dirt or rust has completely disappeared. If not, repeat the treatment with the cleaner again.
Then rub the metal dry with a suitable cloth and check whether the dirt or rust has completely disappeared. If not, repeat the treatment with the cleaner again. - Polish the metal
 Finally, you can make the metal shine again. Special polishing pastes for this purpose are available in specialised shops. If you are dealing with everyday household objects, you can also polish the metal with home remedies: toothpaste (for bronze), lemon peel (for stainless steel) or a mixture of vinegar, flour and salt (for aluminium) are all suitable.
Finally, you can make the metal shine again. Special polishing pastes for this purpose are available in specialised shops. If you are dealing with everyday household objects, you can also polish the metal with home remedies: toothpaste (for bronze), lemon peel (for stainless steel) or a mixture of vinegar, flour and salt (for aluminium) are all suitable.
Cleaning metal for professional use
In the trades or in construction, you won’t get far with home remedies. Metal used for professional applications is rarely used in its untreated state anyway, because surfaces are generally hot-dip galvanised, chrome-plated, silver-coated or powder-coated to increase the corrosion protection and resistance to components and machines. In this case, you can use powerful equipment such as a high-pressure washer to thoroughly clean the metal and prevent further metal corrosion. For painted surfaces, make sure that the water pressure is not set too high.
If a particularly thorough cleaning is necessary (for example, to remove production residues or material abrasion), an ultrasonic cleaner for metal is the best choice. With this, you can remove stubborn residues from metals even in hard-to-reach places. The high-frequency vibrations of the device cause such strong pressure fluctuations that the dirt particles are completely detached from the metal surface after only a few minutes.
How to maintain metal and prevent corrosion
It is exhausting work to clean heavily oxidised aluminium and copper or to remove rust from ferrous metals. The best way to avoid this is to prevent it from happening in the first place by cleaning all metal appliances and surfaces regularly. Every six months or so, carry out a thorough deep cleaning, after which you can impregnate the metal surfaces with a suitable agent.
For this purpose, there are special agents for all metals that have a dirt-repellent effect and, if necessary, provide additional protection against rust and corrosion. To do this, you should regularly remove dirt from any surfaces and check them for damage so that you can counteract any negative effects immediately.
FAQ for cleaning metal
The fact that metal surfaces tarnish, discolour or rust is due to a chemical reaction called oxidation. Depending on the type of metal, oxidation has a negative or protective effect. Non-ferrous metals such as pure aluminium or zinc become particularly corrosion-resistant due to the oxide layer. It only becomes problematic when oxidation promotes corrosion, as is the case with iron and its alloys, for example. Upon contact with water and oxygen, porous rust is formed, which even promotes further corrosion due to its electrochemical properties.
To prevent the corrosion process from progressing and gradually eating away at the material, it is important that you immediately remove the first signs of rust. This will keep your metals stable and durable for a long time.
There are several ways you can make tarnished and dull metal shine again and remove discolouration. Most methods are based on reversing the (electro-)chemical reaction that has previously taken place. Often, treatment with simple household remedies already leads to the desired result.
• For cleaning oxidised metals like aluminium, copper or stainless steel, you need an acid-based liquid. Vinegar, lemon juice, baking soda or cola, mixed with hot water, are sufficient options for this and treat the affected surfaces effectively.
• Oxidised tin can be cleaned with a lye solution. Simply add mild soap or washing-up liquid to warm water and leave the mixture to work on the affected area.
• Zinc is mainly used for everyday objects such as garden tools or gutters. For this reason, it is important to maintain the natural corrosion protection of the patina. Dirt stains should only be removed with water; for heavy soiling, you can use a little rubbing alcohol.
• If silver is tarnished, put it in boiling water with some lemon juice and salt and add a less noble metal (e.g., aluminium foil) to bind the dissolved particles. Make sure that there is sufficient ventilation, as the dissolved sulphur dioxide creates a strong odour.
Do not use cleaners with abrasive particles, hard brushes and scratchy household sponges, as these leave clearly visible scratches on the sensitive surfaces. Soft cotton or microfibre cleaning cloths are best.
Special polishing pastes are available in specialised shops for this. If you are dealing with everyday household objects, you can also polish the metal by using home remedies such as toothpaste (for bronze), lemon peel (for stainless steel) or a mixture of vinegar, flour and salt (for aluminium).
Image source:
gettyimages.de – Photoboyko




















